Research Center
For Patients:
Volunteering for a clinical research study is a critical decision, and you must be informed every step of the way. At Oklahoma City Clinical Research Center, we would like to provide you with information to help you make the right decision. Our team has participated in and continues to research the use of cutting-edge treatments and medical devices, and we are dedicated to advancing medical research across various fields.
Our Research Areas
At Oklahoma City Clinical Research Center, we are committed to advancing medical treatments through clinical trials. Below are some of the key areas of research that we currently focus on:
Spinal Health and Instability
We are conducting extensive research in the treatment of spinal instability, working with the latest medical devices and therapies to improve patient outcomes. Our studies aim to develop innovative solutions for treating conditions affecting the spine and related musculoskeletal systems.Nephrology Research
We are expanding our footprint into nephrology, seeking to collaborate with researchers and industry partners to advance treatments for kidney diseases. Our focus is on driving innovation in kidney health, improving treatment options, and enhancing patient care.Endocrinology Research
Our clinical trials also focus on endocrinology, exploring treatments for hormonal disorders, metabolic diseases, and thyroid conditions. Through this research, we aim to uncover new therapies and refine existing ones, ultimately improving the quality of life for patients with these conditions.
Safety and Ethical Standards
At Oklahoma City Clinical Research Center, patient safety is our top priority. All clinical trials are conducted in strict accordance with regulatory guidelines to ensure participant well-being. Our team of researchers and healthcare professionals closely monitors every aspect of each study to maintain the highest standards of care.
In addition to prioritizing safety, ethical conduct is at the core of our operations. We uphold transparency, respect, and integrity in all our interactions with participants, sponsors, and regulatory authorities. Every study is designed and conducted with the utmost professionalism, ensuring that we maintain the trust of our participants and contribute valuable data to the scientific community.
For Sponsors/CRO'S:
Oklahoma City Clinical Research Center has been in operation since 2018 under the direction of owner and principal investigator Cheng-Lun Soo, MD. Dr. Soo has been a practitioner for over 25 years. He is board-certified in Orthopedic Surgery and is president of The Oklahoma Center for Spine and Pain Solutions here in Oklahoma City. He has served as a sub-investigator and principal investigator on clinical trials since 1999. His dedication to the industry has been most valuable in overseeing 49 clinical research trials.
Our staff includes a site director who oversees the quality of operations at the site, manages our regulatory department and oversees the study start-up process. We also have a study coordinator, X-ray technicians, and other administrative staff
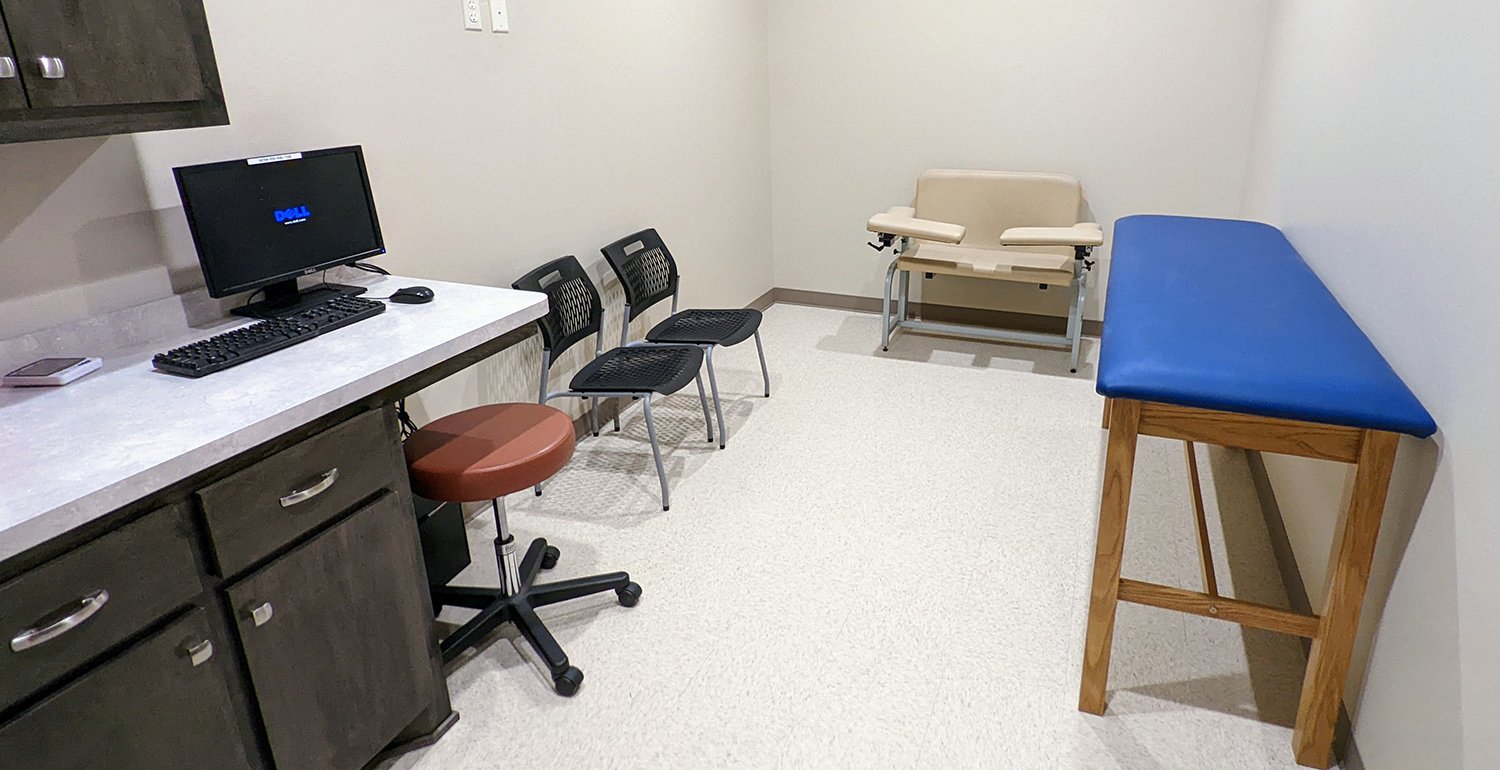
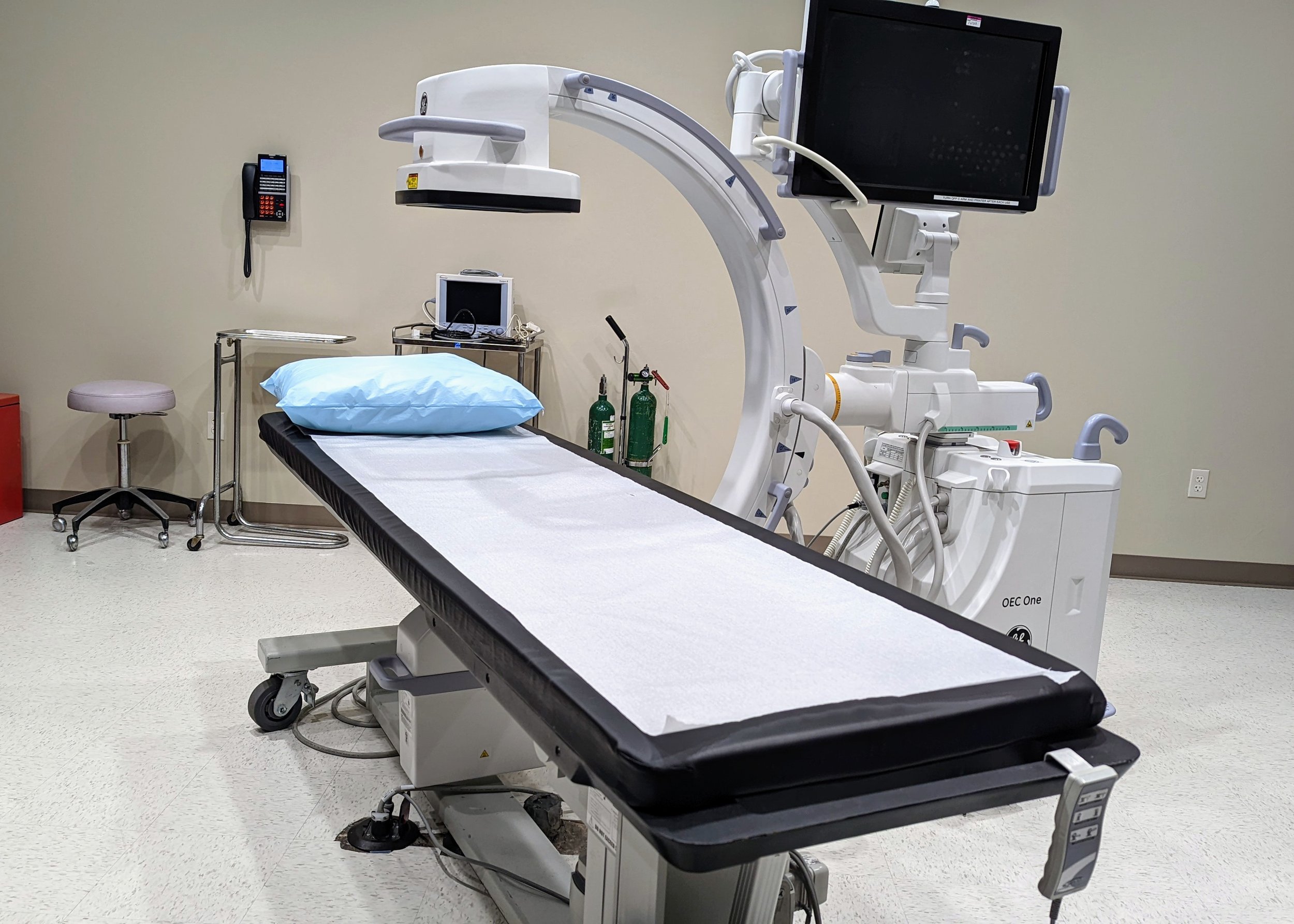
The facility at Oklahoma City Clinical Research Center includes a manual and automated vital sign apparatus, min/max temperature-controlled refrigerator and -20 freezer, all of which are calibrated annually. Our independent, double-locked, ambient drug room stores investigational products and controlled substances. There is an independent lab with a documented CLIA waiver with plenty of storage for lab supplies. We have two large rooms for monitoring visits with WIFI access, a conference room for meetings and multiple exam rooms for informed consent and study visits. In addition, Oklahoma City Clinical Research Center has the capability for X-ray services.
Our site experience includes working with many sponsors and CRO's in the industry. Our data experience includes InForm, iMedidata/RAVE, Oracle and more. We have also collaborated with such companies as Acurian and StudyKik to assist patient recruitment.
Our commitment to providing our sponsors and vendors with the highest quality data is our ultimate goal. In addition, our mission is to provide study volunteers with the latest and most effective alternative treatments while assuring their health and safety in clinical trials.
Facility Overview
Our facility is equipped with state-of-the-art technology, including manual and automated vital sign apparatus, temperature-controlled storage for investigational products, and an independent lab with a CLIA waiver. We provide a secure and compliant environment for clinical trials, with dedicated rooms for patient visits, informed consent discussions, and sponsor meetings.
If you're interested in participating in an upcoming trial or need additional information, please email our site representative, Lupita Alvarez, via email at business@okclinicalresearch.com.
References:
Clinical Research. University of Virginia School of Medicine. (2020, October 26). Retrieved February 23, 2022, from https://research.med.virginia.edu/clinicalresearch/what-is-medical-research/#:~:text=Clinical%20research%20is%20the%20study,prevent%2C%20diagnose%20and%20treat%20illness.&text=Simply%20put%2C%20it%20involves%20human,and%20information%20to%20benefit%20patients.